SCIENCE & TECHNOLOGY
Vision for the Future
| Candidate | Indication | Preclinical | Clinical | IP | Remarks |
|---|---|---|---|---|---|
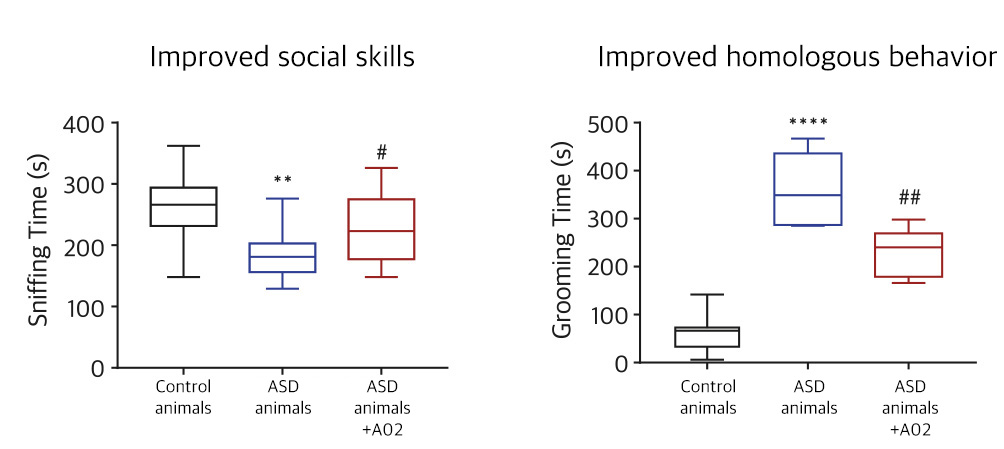
| NV01-A02 | Autism spectrum disorder |
Proceeding
|
KR, PCT, USA, EU, China, Japan | * Domestic clinical trial phase 2 in progress | |
| Fragile X syndrome |
Proceeding
|
KR, PCT, USA, EU, China, Japan | * Preparing for phase 2 overseas clinical trials | ||
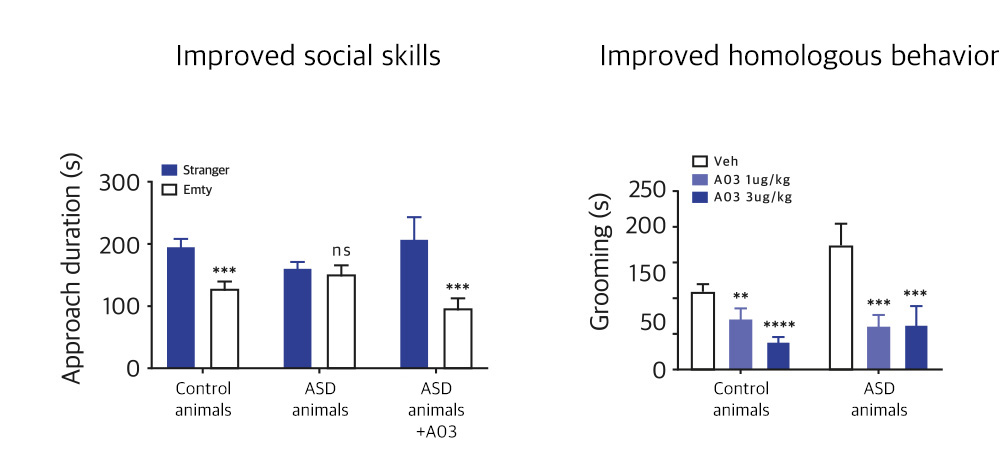
| NV01-A03 | Autism spectrum disorder |
Proceeding
|
KR, PCT, USA, EU, China, Japan | * Preparing for phase 2 overseas clinical trials | |
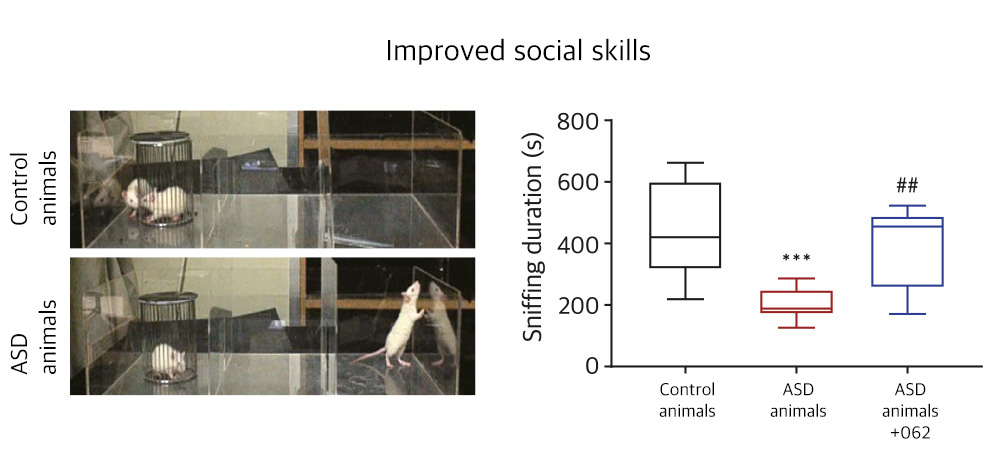
| NV01-062 | Autism spectrum disorder |
Proceeding
|
KR, PCT | ||
| NV01-E01 | Autism spectrum disorder |
Proceeding
|
* Exploratory clinical trials in progress | ||